Department of Physical Chemistry
Nanostructured Model Catalysts

Department of Physical Chemistry
Nanostructured Model Catalysts
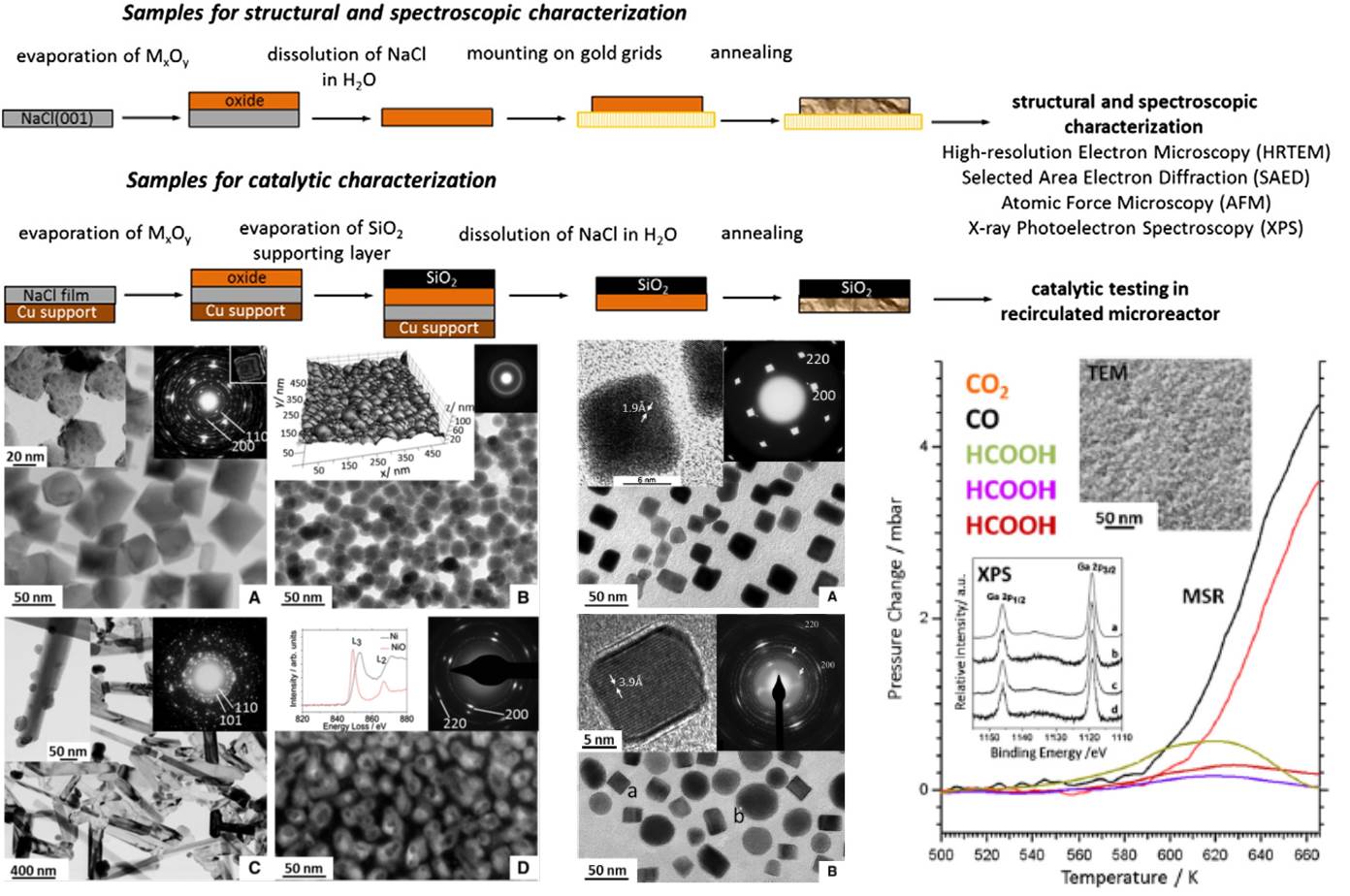
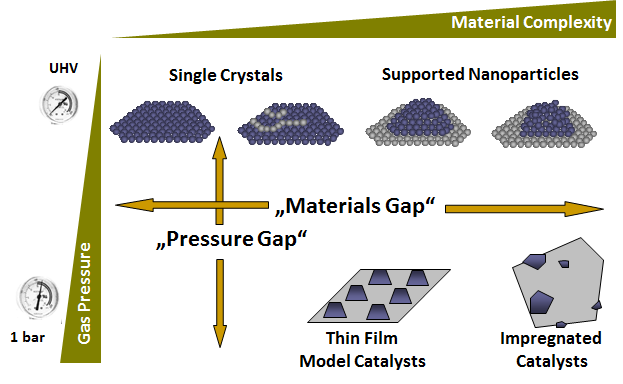
This part of research is very unique as it exploits a characteristic facet of the NaCl structure, namely (001), to grow well-ordered thin films of oxides, metal-oxide and intermetallic-oxide films. The reason for preparation of such a system lies in the still existing pressure and material’s gap in catalysis, which is schematically shown below. In short, model systems (such as the discussed thin films) are necessary, since structurally highly complex materials employed in most technological applications can normally not be used in mechanistic studies of chemical or catalytical reactions. These materials usually exhibit structurally and electronically different sites with varying sizes and morphologies (“material’s gap”). To gain mechanistic insight, however, uniformity with respect to structure of active sites is required. The thin film model systems allow to bridge this gap, since they are well-ordered, but still complex enough to mimic technologically relevant materials. A second advantage lies in the ability to also bridge the so-called “pressure gap”, meaning that model systems can only be studied in pressure regimes (usually UHV conditions with p < 10-10 mbar) that are far away from those used in catalysis technology (bar-regime). Thin films can be studied under realistic conditions using a dedicated kientic microreactor setup. Generally, due to their high ordering and small film thicknesses, the films are perfect and dedicated samples for electron microscopy investigations.
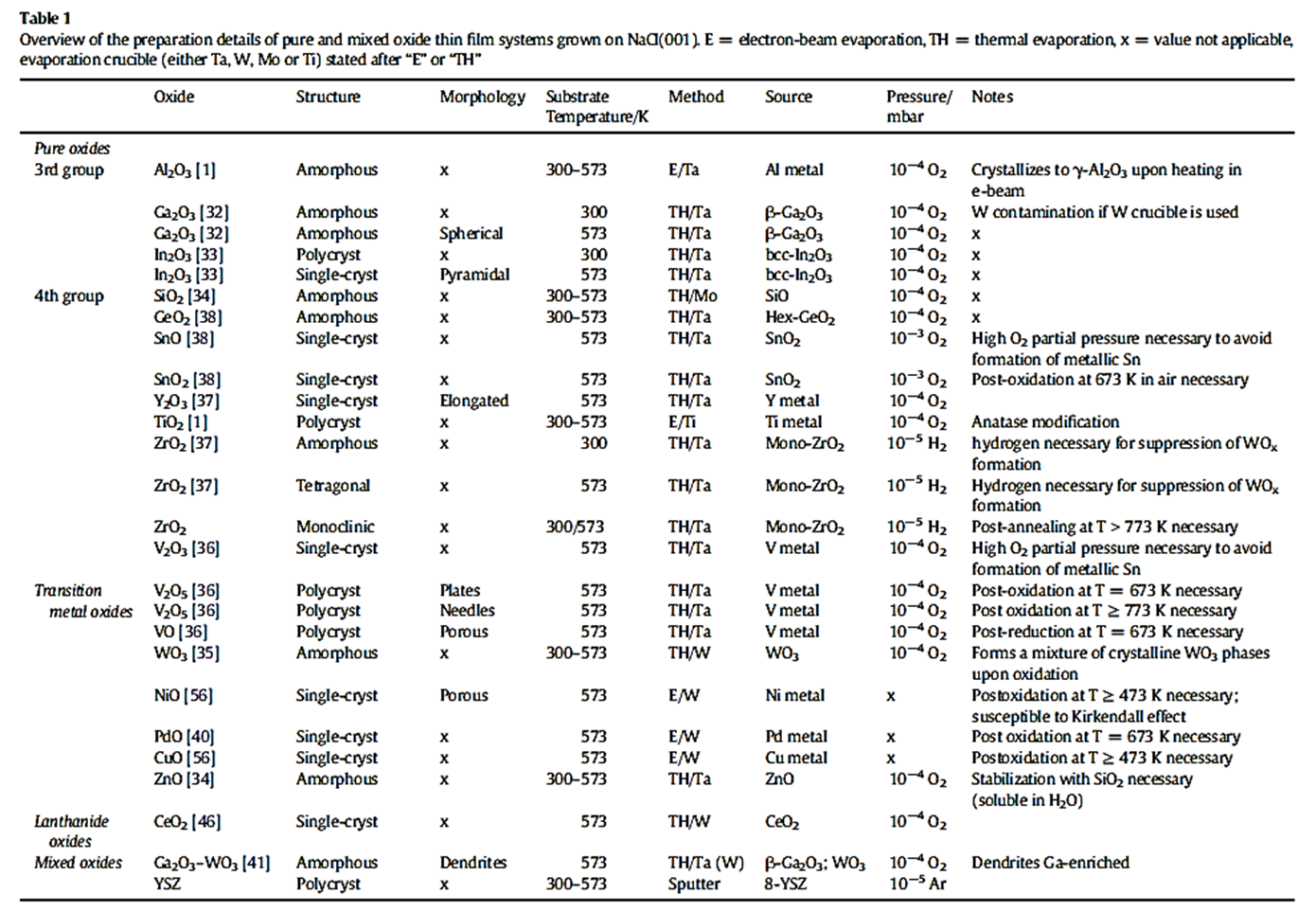
The variety of systems that can be prepared is extraordinarily widespread and encompasses binary and more complex oxides, metal-oxide systems or intermetallic-oxide systems. Table 1 gives an overview just of oxidic materials that have recently been prepared, characterized and tested. Especially for the metal-oxide systems, the model systems unfolds its particular strenghts: it usually creates a large metal-oxide interface, which is particular useful in catalysis (see Research Area 3).