Department of Physical Chemistry
Nanostructured Model Catalysts

Department of Physical Chemistry
Nanostructured Model Catalysts
This part of our research is entirely devoted to studies on nanostructured oxide materials. Although an important class of materials with frequent applications in technology or catalysis, the comples surface and bulk chemistry makes the correlation between structural changes occuring during use and the nature of the chemical site responsible for these changes difficult. Such structural changes (and the associated alterations in morphology or electronic structure) might include, but are not limited to: alternating surface or bulk structural terminations, simultaneous presence of crystallinr and amorphous phases, presence of different oxidation states or structural reconstructions. Furthermore, the picture of oxides is further complicated by the intrinsic polymorphism of many oxidic materials, even more so since most of these metastable polymorphs can be stabilized in their respective nanostructured versions.
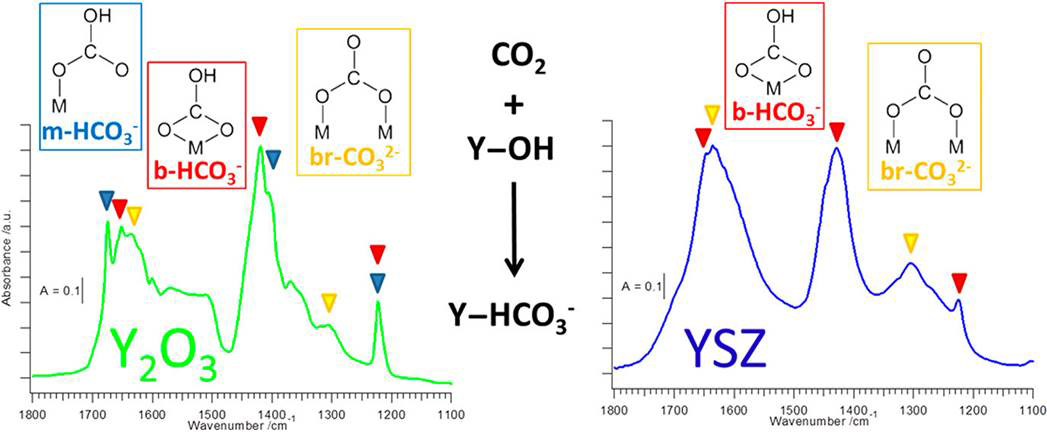
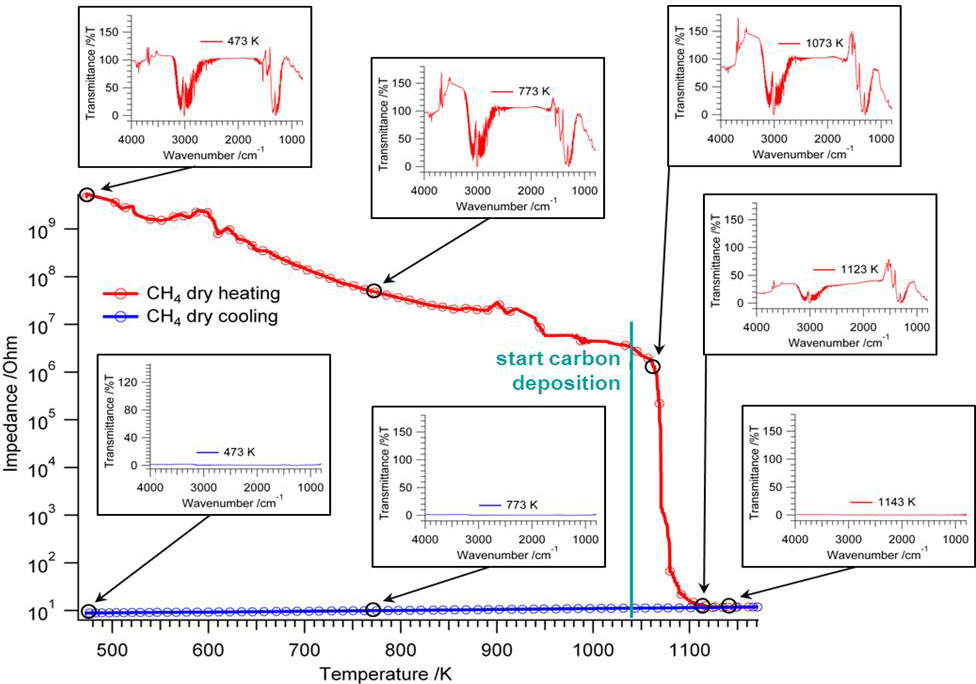
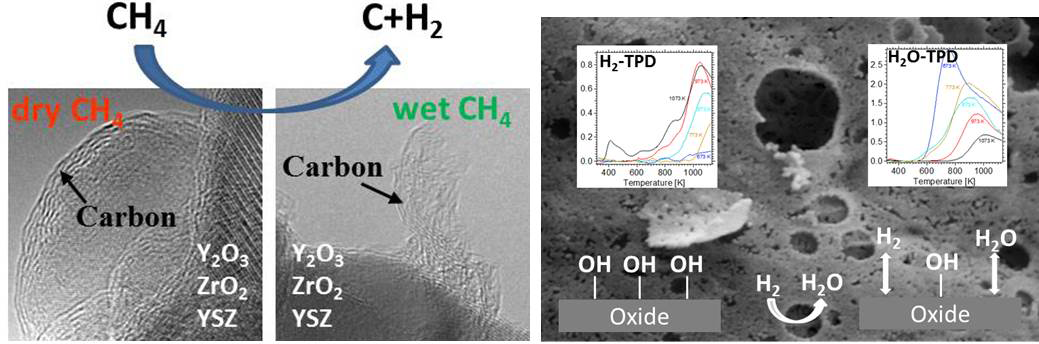
Our aim is to add to the understanding of their physico-chemical properties by a combined approach of structural, electronic or catalytic characterization using different methods: these include electron microscopy techniques, (high-temperature) X-ray diffraction, spectroscopic characterization (operando FT-IR, Raman, electric impedance spectroscopy), volumetric adsorption techniques (temperature-programmed desorption,- reaction or -reduction), catalytic characterization (e.g. reforming reaction, water-gas shift equilibrium). We are also able to study the surface structure of these materials using dedicated methods, such as AFM or ion scattering. This allows us to separately study the surface and bulk structural properties alongside their defect chemistry. Especially the latter in many cases dominates the intrinsic reactivity of the individual oxides.
For these studies we frequently employ our thin film model systems, which is in detail discussed below, and can be used to nanoarchitecture different oxide particle morphologies. Recently, our research focus also shifted to beyond “simple” binary oxides to more complex pure oxidic materials of higher technologies such as perovskite-based or spinel-type oxides.