Department of Physical Chemistry
Nanostructured Model Catalysts

Department of Physical Chemistry
Nanostructured Model Catalysts
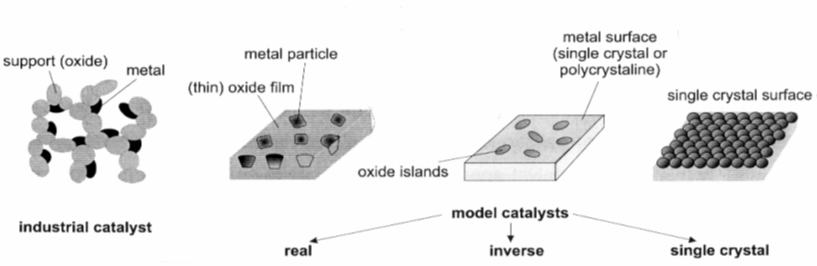
This research area very much forms the core and center of our expertise and not only gets input from all the other research areas, but focusses them on the important question of how to determine the active and selective site of catalytic entities. Most of them exhibit phase boundary regions - either deliberately or formed in-situ during the catalytic reaction - and it is this region, which is most important for catalysis. In a bifunctional synergistic way, the reaction can be facilitated either by special catalytic sites located directly at the phase boundary (i.e. they are sites of the boundary region themselves) or by enhanced transport and exchange of reactants across the phase boundary from one phase (e.g. a metal) to another (e.g. an oxide). There are different ways to tackle the problem of how to determine the influence of the interfacial contact region. One particular promising way is to use differently prepared model systems with different extent of interfacial region and to compare the structural and catalytic behavior. These might include thin film systems (see above) with embedded metal particles in an oxide matrix, conventionally wet-chemical impregnated powder systems or so-called inverse model systems. These are (sub-)monolayers of oxides deposited on noble metal surfaces and are particularly useful in adjusting the metal-oxide interfacial region. Recent research was focussed on the extension of this concept to more comples systems, such as those of alloy or intermetallic-oxide systems. Common to all inverse model systems is the typical preparation under UHV conditions at very low pressures (< 10-10 mbar), allowing surface science investigations of the (electronic) struacture of those materials before and after catalytic use. As it is necessary to bridge the so-called materials and pressure gaps, our setup not only allows this surface science studies, but also catalytic testing under realistic conditions using an attached catalytic reactor cell. The sample itself can be conveniently transferred between the main chamber (for surface science studies) and the catalytic cell. One particular advantage of this approach is the possibility to also study these samples under in-situ and operando conditions at special reseach facilities such as synchrotron light sources. The latter allow spectroscopic and structural characterization during reaction – that is, in the working state of the catalysts. Typical employed methods include in-situ X-ray photoelectron Spectroscopy (XPS) or X-ray absorption spectroscopy (XAFS).
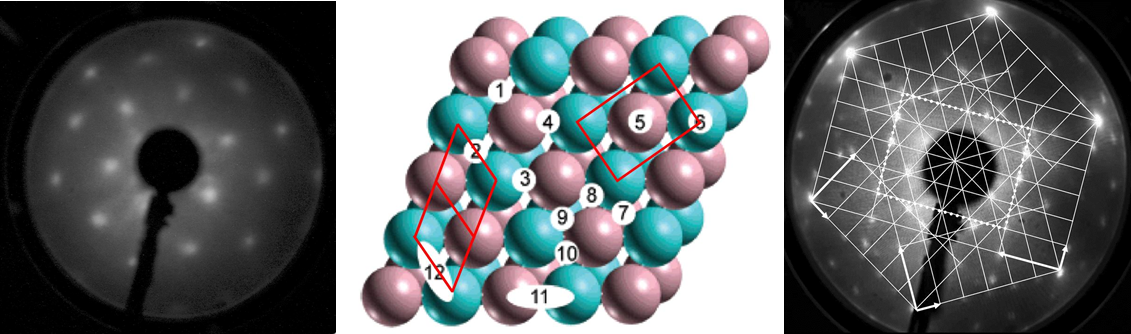
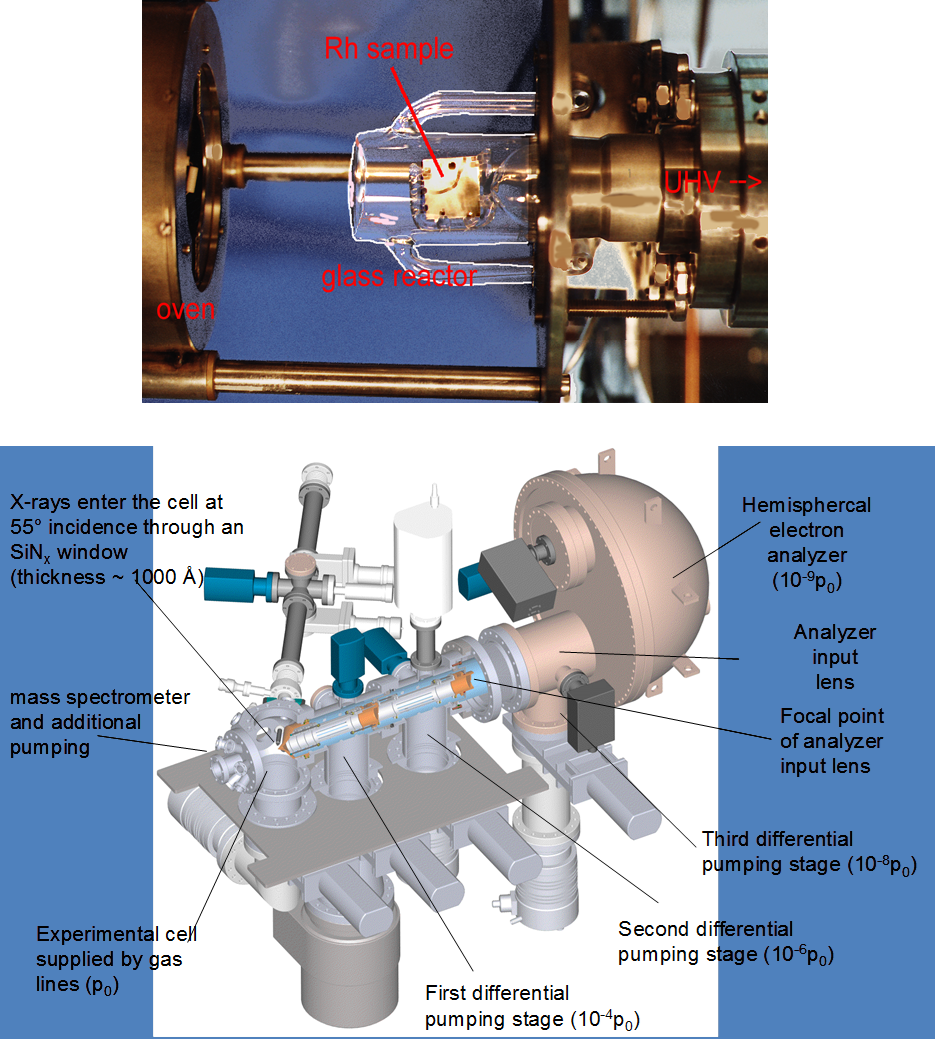
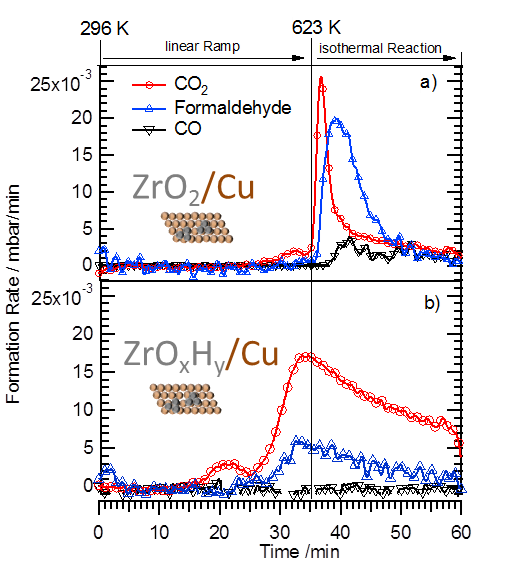
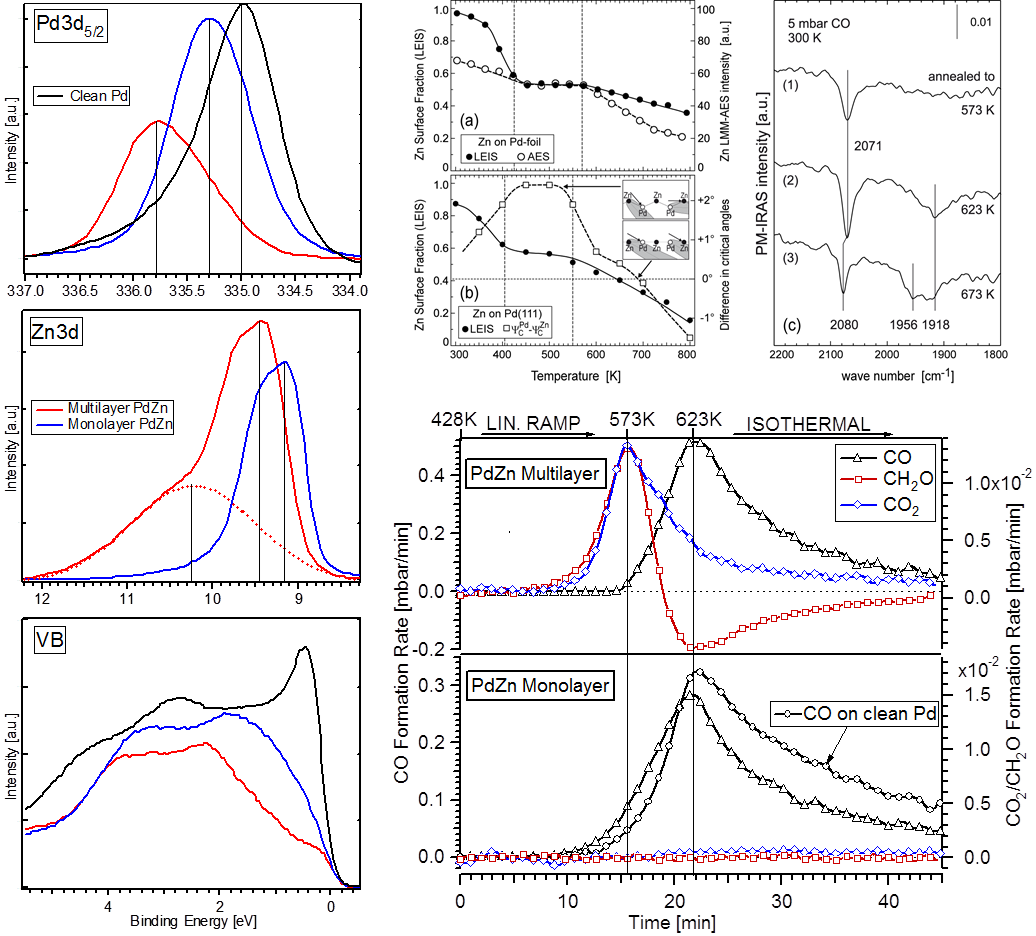
Within the last years the structural and catalytic characterization of a set of different metal-oxide and oxide-metal, as well as binary metallic model-oxide systems has been carried out. The latter include bimetallic subsurface alloys as well as three-dimensional surface alloy phases (e.g. Pt-V, Pd-V, Pt-Ce, Pd-Zn, Pd-Ga, Pd-In or Cu-Zn), both studied on single and polycrystalline surfaces. These were characterized by a combination of surface science techniques, and by adsorption and high pressure catalytic studies (methanol and methane steam reforming, water-gas shift equilibrium, CO hydrogenation). Also the transition from sub-monolayers of reducible oxides deposited on noble metal surfaces (the so-called “inverse model catalysts”) toward bimetallic surface phases was scrutinized. Complementary to the inverse models, thin film-supported bimetallic model catalysts (“real” model systems such as Pt-CeO2, Pt-SiO2, Pt-Al2O3, Rh-CeO2, Rh-V2O3, Pd-ZnO, Pd-Al2O3) were prepared by reductive activation, and the induced structural and catalytic effects were studied by (high-resolution) transmission electron microscopy and test reactions such as those mentioned above.